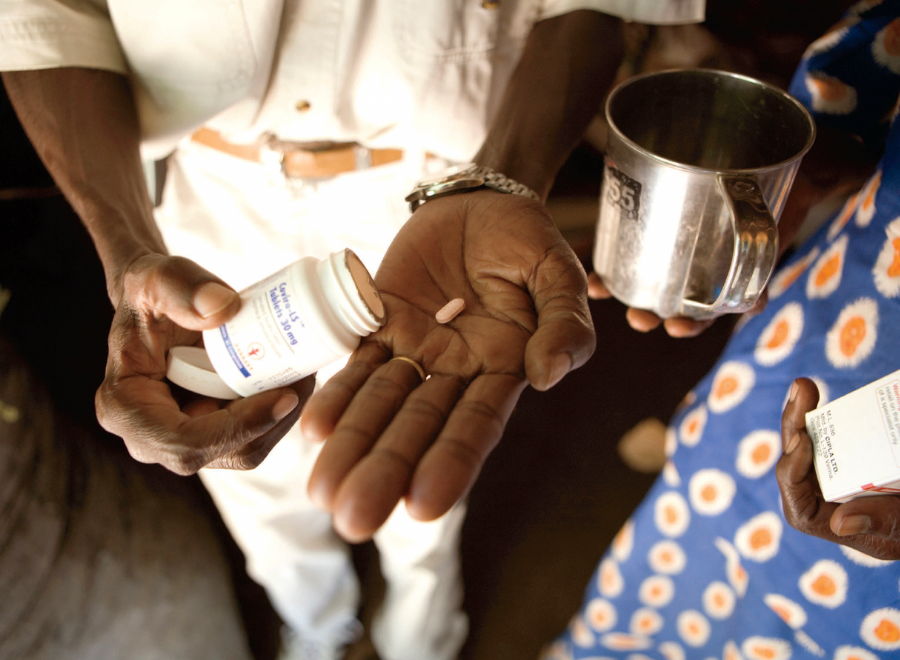
It was the right thing to do: passing a law that would help put affordable medication into the hands of people suffering from HIV-AIDS. Patients would live longer, healthier lives. Drug companies would still profit. But what started as an act of pharmaceutical goodwill has been watered down — if not discreetly shelved and abandoned.
In 2004, the Parliament of Canada responded to the urgent need for anti-AIDS drugs in many developing countries by creating Canada’s Access to Medicines Regime (CAMR). With wide support from political parties and civil society groups, including The United Church of Canada, the government promised access to the latest therapies at significantly reduced prices by encouraging brand-name pharmaceutical firms to allow generic ones to manufacture cheaper versions of their anti-AIDS medications. Described by NGOs as “a gift tightly bound in red tape,” however, the law has been used but once in Canada. In fact, the single generic company involved in the process vowed never to repeat it.
You may unsubscribe from any of our newsletters at any time.
The reason isn’t as complex as the law itself. Brand-name pharmaceutical companies, concerned about patent protection and increased competition at home and abroad, saw to it that their generic counterparts must await approvals for each shipment of medication — a process that takes months if not years. As a result, CAMR continues to come short of its promise and leaves AIDS activists wondering if Canada will ever make universal access to treatments a reality.
“Quite simply, the resources needed to scale up AIDS treatment will have all the more impact if countries can buy the medicines they need at lower prices,” says Richard Elliott, executive director of the Canadian HIV/AIDS Legal Network.
The network, along with a number of Canadian activists and politicians, has been working to fix the flaws of CAMR. Bill C-393, a private member’s bill that would streamline the regime, is currently before Parliament. It outlines a one-licence process, whereby the government would authorize a generic company — right from the beginning — to handle all of its generic drug production. This would do away with separate negotiations and licensing for every single order of medicine by individual countries.
“What we have said to the federal government is that you can’t do things on a case-by-case basis, drug order by drug order, country by country,” Elliott says. “That doesn’t make much sense for the generic producer nor for the developing country that wants to buy.”
Reversing the spread of HIV-AIDS is possible. Mass prescription of anti-retroviral treatments (ARTs) could wipe out the disease by the middle of this century, according to the South African Centre for Epidemiological Modelling and Analysis. It’s part of a growing body of scientists who argue that without an effective vaccine, ARTs offer the best hope of preventing — and even eliminating — AIDS. ARTs, which must be taken daily, dramatically lower the concentration of HIV in a person’s bloodstream and, in addition to preventing the patient from developing AIDS, significantly reduce their ability to transmit the virus to another person.
Of course, no one country has the capacity to meet the global demand for these drugs. Indian generic manufacturers, most notably, have supplied more than 80 percent of donor-funded AIDS medicines to developing countries in the last seven years. But as Elliott points out, intellectual property rights agreements, administered by the World Trade Organization, have curtailed India’s production of second-generation medicines. It’s not clear whether the country can continue supplying generic versions of these newer and improved ARTs.
“We can’t keep viewing India, for example, as the global pharmacy of the poor,” Elliott says. “The picture is changing and will continue to get worse over the coming years.”
To date, the only Canadian company to deliver affordable drugs has been Apotex Inc., Canada’s largest generic pharmaceutical firm, which produces 260 medicines for 115 countries. But it took the corporation four years to make its first — and only — shipment of medicines. In September 2009, it sent a three-in-one AIDS drug, Apo-TriAvir, to Rwanda. In a May 2009 press release, Apotex president Jack Kay reiterated the need to fix CAMR. “The regime has not been successful in attaining its original objectives as declared when the law creating it (the Jean Chrétien Pledge to Africa) was passed,” he said. “We invested millions in the research and development of the product, legal costs in negotiating with the brand companies and made no profits in this process. In its current form, the law is not workable for us and, it appears, it doesn’t work easily for developing countries.”
In the absence of affordable anti-retrovirals, many countries, private donors and charities have heeded the call for change. This past September, Prime Minister Stephen Harper pledged $30 million to combat AIDS and other diseases at a UN anti-poverty summit. Canada promised to give another $540 million toward the same between 2011 and 2013, which represents a 20 percent increase on its commitment of $450 million over the previous three years. The world’s wealthiest countries have to forge “practical, durable solutions,” Harper said, and maintain“a shared sense of responsibility.”
Although increased AIDS funding this past decade has helped many of the nearly 34 million people living with HIV-AIDS around the world, there are still more than nine million — mostly in Africa — who need access to life-saving ARTs and cannot get them, according to Dignitas International, a Toronto-based medical humanitarian organization that treats thousands of AIDS patients in Malawi.
In a June opinion piece published in the Globe and Mail, Dignitas founders James Orbinski and James Fraser, both formerly of Médecins Sans Frontières (MSF), pondered if the world had forfeited victory in the battle with AIDS: “G8 nations committed to ensuring universal access to HIV/AIDS treatment, and their performance on this promise has saved millions of lives. Although laudable, the gains made over the past decade are less than what should have been achieved, and are now threatened by a retreat of these nations from their funding commitments.”
As is the case in other countries with a high prevalence of HIV, Malawi’s shortages of anti-retroviral drugs are partly due to delayed disbursements from the Global Fund to Fight AIDS, Tuberculosis and Malaria, according to Orbinski and Fraser. The international fund was established in 2002 to finance interventions against pandemics. But in these recessionary times, many of the world’s biggest donors have frozen or reduced their contributions to anti-AIDS treatments in particular. This has affected patients in Malawi twofold: Dignitas’s medical team can no longer take on new AIDS patients in its rural clinics, ensuring that only the absolute sickest — and most “immuno-compromised” — continue with their regimen. Secondly, those on treatment must travel up to 40 kilometres to the southern city of Zomba, where they receive a one-month supply of medicine at the central hospital.
Orbinski and Fraser described an environment where patients, distraught and exasperated, blame their nurses and clinicians. “They’re angry at us because we’re at the front line.” For patients aware of anti-retroviral medicine, the scarcity of treatment feels crueler and more inexplicable, the Dignitas founders wrote.
Their call for action is echoed by Rachel Kiddell-Monroe, the president of Universities Allied for Essential Medicines, an international organization working to improve access to medicine in low-income and lower-middle-income countries. With MSF Canada, Kiddell-Monroe also witnessed first-hand the impact of the pandemic in the absence of affordable anti-retrovirals. “I saw how men and women in Rwanda after genocide were left to die because there were no AIDS treatments available to them. Quite simply, the prices of drugs were too high.”
The major difficulty, she says, has been the “incredible power of the pharmaceutical lobby in Canada and its insistence on returning to diversionary arguments.”
The brand-name pharmaceutical industry once labelled CAMR “window dressing.” It argued that in addition to eroding patent protection, low-cost generics could be smuggled back to North America, undercutting the more expensive brand-name drugs. With more competition — and a lesser payday — there are fewer incentives to research and develop new medicines, Canadian companies say.
To make CAMR palatable to the pharmaceutical industry, some members of Parliament made sure that generic companies had to undergo a stringent application process for each shipment. But Kiddell-Monroe says their willingness to appease brand-name pharmaceuticals shows “either a real lack of understanding of international development issues or a wilful disregard for our commitments as Canadians — and as human beings — in favour of protecting an already lucrative drug industry…. So the fundamental flaw in the current legislation is that industry profit-seeking trumps the rights of patients.”
A single pill costing 15 cents to make can end up retailing for $1.50, according to Elliott. If GlaxoSmithKline, for example, has a patent on one anti-AIDS drug while Abbott and Pfizer have one on others, those three brand-name competitors won’t get together to produce a cheap, fixed dose that combines their three different drugs. It’s not good for their bottom line.
Says Elliott, “Generic drugs, like Apotex’s three-in-one AIDS pill, are the key, but not just on the pricing issue. Such manufacturers have the ability to produce products that make dosing simpler and therefore increase people’s ability to adhere, especially where infrastructure is limited.”
Canada has long been regarded as a “constructive, compassionate and humanitarian” actor on the global stage, Elliott says. But this image has been tarnished in recent years, partly due to its failed AIDS initiative, as well as the lack of progress toward the 2015 Millennium Development Goals, a set of anti-poverty targets set by UN countries that includes combatting HIV-AIDS.
“It’s a widely shared belief among Canadians that we should make HIV-AIDS medicines available and affordable to developing countries,” Elliott says. “We understand that we have the capacity to help save millions of lives and that we simply can’t wall off the public health problems that arise elsewhere in the world.
“Bill C-393, if passed, will finish what the country started in 2004. It’s a win for patients in the developing world; a win for Canadian generic companies that can supply those medicines; a win for brand-name drug companies that would receive royalties; and a win for Canada’s international reputation — all at no cost to Canadian taxpayers.”
***
This story first appeared in The United Church Observer’s December 2010 issue with the title “Prescription for a pandemic.”